Determine the number of protons, neutrons, and electrons in the following: 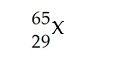
A) p+ = 36 n° = 29 e- = 36
B) p+ = 29 n° = 29 e- = 36
C) p+ = 36 n° = 36 e- = 29
D) p+ = 29 n° = 36 e- = 29
E) p+ = 29 n° = 36 e- = 36
Correct Answer:
Verified
Q22: Identify the symbol for fluorine.
A) F
B) Fl
C)
Q22: Predict the charge that an aluminum ion
Q28: Which of the following statements about isotopes
Q29: Identify the symbol for silver.
A) S
B) Si
C)
Q36: How many protons are in arsenic?
A) 33
B)
Q38: Predict the charge that a calcium ion
Q39: Which of the following statements about subatomic
Q41: The atomic mass of tungsten is 183.84
Q42: Carbon has two naturally occurring isotopes, carbon-12
Q43: Bromine has two naturally occurring isotopes, Br-79
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents