A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 0.8386 bar. Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 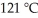 while maintaining the pressure at 0.8386 bar.
while maintaining the pressure at 0.8386 bar.
A) 10.9 L
B) 13.2 L
C) 2.07 L
D) 7.56 L
E) 48.4 L
Correct Answer:
Verified
Q47: Which of the following statements is TRUE?
A)
Q62: A sample of N2 effuses in 255
Q101: A container filled with gas is connected
Q103: What is the definition of diffusion?
A) gas
Q112: What is the temperature of NO2 gas
Q113: You have three cylinders containing O2 gas
Q121: Which of the following would have a
Q123: Three identical flasks contain three different gases
Q124: A gas bottle contains 0.250 mol of
Q131: Give the formula for ozone.
A) O3
B) O2
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents