Multiple Choice
Which of the following substances (with specific heat capacity provided) would show the greatest temperature change upon absorbing 100.0 J of heat?
A) 10.0 g Ag, CAg = 0.235 J g-1 °C-1
B) 10.0 g H2O, 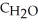 = 4.184 J g-1 °C-1
= 4.184 J g-1 °C-1
C) 10.0 g ethanol, Cethanol = 2.42 J g-1 °C-1
D) 10.0 g Fe, CFe = 0.449 J g-1 °C-1
E) 10.0 g Au, CAu = 0.128 J g-1 °C-1
Correct Answer:
Verified
Related Questions
Q4: A piece of iron (C = 0.449
Q9: Calculate the amount of heat (in kJ)
Q12: Calculate the amount of heat (in kJ)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents