Choose the best Lewis structure for OCl2.
A) 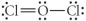
B) 
C) 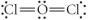
D) 
E) 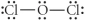
Correct Answer:
Verified
Q27: The phosphorus atom in PCl3 would be
Q42: Give the number of valence electrons for
Q68: Which of the following ionic compounds would
Q81: Of the following elements,which has the highest
Q91: The electronegativity is 2.1 for H and
Q103: Choose the best Lewis structure for SeO42⁻.
A)
Q103: The compound ClF contains:
A)ionic bonds
B)nonpolar covalent
Q108: How many of the following elements can
Q143: Which ionic compound would be expected to
Q152: Which of the following ionic compounds would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents