Consider the molecule below. Determine the molecular geometry at each of the three labelled atoms. 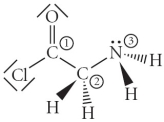
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal
Correct Answer:
Verified
Q12: Place the following in order of increasing
Q23: Determine the electron geometry (eg),molecular geometry (mg),and
Q34: A molecule containing a central atom with
Q35: Determine the electron geometry (eg),molecular geometry(mg)and polarity
Q39: Choose the compound below that contains at
Q57: A pilot checks for water in the
Q61: Determine the electron geometry (eg), molecular geometry
Q64: Determine the electron geometry (eg), molecular geometry
Q66: Determine the electron geometry (eg), molecular geometry
Q74: A molecule containing a central atom with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents