Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 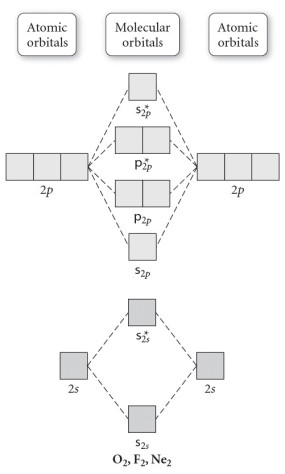
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above is paramagnetic.
Correct Answer:
Verified
Q55: Draw the Lewis structure for BrCl3.What is
Q56: Consider the following compound.How many sigma and
Q100: What is the molecular geometry of NCl3?
A)T-shaped
B)tetrahedral
C)trigonal
Q115: Use the molecular orbital diagram shown to
Q117: Draw a molecular orbital diagram and use
Q127: A molecule or ion with eight electrons
Q128: A molecule or ion with three electrons
Q129: Draw a molecular orbital diagram and use
Q133: In molecular orbital theory the acronym LUMO
Q148: Using the VSEPR model, the molecular geometry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents