Use the molecular orbital diagram shown to determine which of the following is least stable. 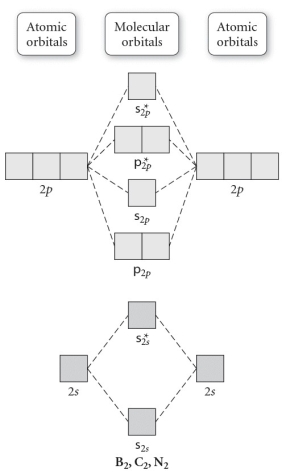
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B21⁺
Correct Answer:
Verified
Q71: Draw the Lewis structure for the molecule
Q101: Draw the Lewis structure for the molecule
Q111: Use the molecular orbital diagram shown to
Q113: Use the molecular orbital diagram shown to
Q115: Use the molecular orbital diagram shown to
Q127: A molecule or ion with eight electrons
Q128: A molecule or ion with three electrons
Q129: Draw a molecular orbital diagram and use
Q131: Draw a molecular orbital diagram and use
Q140: A molecule or ion with two electrons
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents