Assign the appropriate labels to the phase diagram shown below. 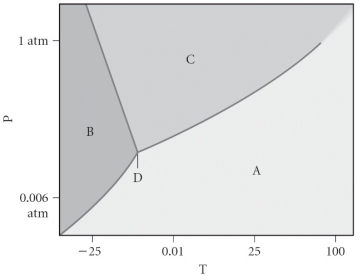
A) A = liquid, B = solid, C = gas, D = critical point
B) A = gas, B = solid, C = liquid, D = triple point
C) A = gas, B = liquid, C = solid, D = critical point
D) A = solid, B = gas, C = liquid, D = supercritical fluid
E) A = liquid, B = gas, C = solid, D = triple point
Correct Answer:
Verified
Q49: At atmospheric pressure,dry ice
A) freezes.
B) deposits.
C) sublimes.
D)
Q54: Why is water an extraordinary substance?
A) Water
Q67: The freezing point of water is _.
A)
Q71: Consider the phase diagram shown. Choose the
Q72: Determine the radius of an Al atom
Q76: Identify triple point.
A) the temperature, pressure, and
Q77: Vanadium crystallizes in a body-centred cubic structure
Q79: Which of the following is considered an
Q79: How much energy must be removed from
Q83: Identify the compound with the highest boiling
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents