Express the equilibrium constant for the following reaction: 2 CH3Cl(g) + Cl2(g) ⇔ 2 CH2Cl2(g) + H2(g)
A) Kp = 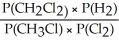
B) KP = 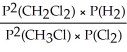
C) KP = 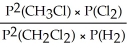
D) KP = 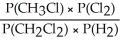
E) KP = 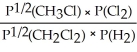
Correct Answer:
Verified
Q1: Give the direction of the reaction if
Q1: Give the direction of the reaction if
Q10: The equilibrium constant is given for one
Q15: Express the equilibrium constant for the following
Q16: Express the equilibrium constant for the following
Q23: For which reaction will Kp = Kc?
A)S(s)+
Q24: Express the equilibrium constant for the following
Q27: For which of the following reactions will
Q31: For the following reaction, what is Δn
Q39: For the following reaction, what is Δn
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents