Express the equilibrium constant for the following reaction: 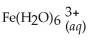 + 6CN-(aq) ⇌
+ 6CN-(aq) ⇌ 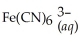 + 6H2O(l)
+ 6H2O(l)
A) Kc = 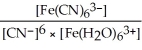
B) Kc = 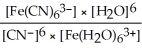
C) Kc = 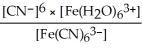
D) Kc = 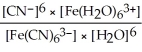
E) Kc = 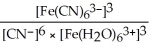
Correct Answer:
Verified
Q21: For the following reaction, what is Δn
Q29: Express the equilibrium constant for the following
Q31: Express the equilibrium constant for the following
Q36: Express the equilibrium constant for the following
Q38: The reaction below has a Kc value
Q39: The reaction below has a Kc value
Q45: For the following reaction, what is △n
Q48: For the following reaction, what is △n
Q51: Determine the value of Kc for the
Q56: For the following reaction, what is △n
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents