Find the correct equilibrium constant that shows the ionization constant of a weak acid HA.
A) Ka = 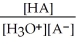
B) Ka = 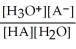
C) Ka = 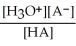
D) Ka = 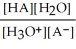
E) Ka = 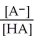
Correct Answer:
Verified
Q7: Which of the following is a weak
Q23: On which equilibrium does the strength of
Q25: What is the Kw of pure water
Q26: Calculate the pH of a solution that
Q28: The stronger the acid, _.
A) the stronger
Q30: Calculate the concentration of H3O⁺ in a
Q34: Calculate the concentration of OH⁻ in a
Q37: Which of the following equilibria represents the
Q37: Which of the following solutions would have
Q38: Which of the following is correct?
A) The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents