Determine the ammonia concentration of an aqueous solution that has a pH of 11.00. The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 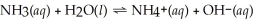
A) 3.0 mol L-1
B) 0.056 mol L-1
C) 1.8 × 10-2 mol L-1
D) 1.0 × 10-3 mol L-1
Correct Answer:
Verified
Q121: Which one of the following salts,when dissolved
Q126: Which one of the following salts,when dissolved
Q129: Which one of the following salts, when
Q130: If an equal number of moles of
Q133: Match the following.
-Lewis acid
A)proton donor
B)electron pair acceptor
C)proton
Q133: Which one of the following salts,when dissolved
Q135: What is the selenide ion concentration [Se2-]
Q136: Calculate the concentration of bicarbonate ion, HCO3-,
Q138: Match the following.
-Lewis base
A)proton donor
B)electron pair acceptor
C)proton
Q139: The acid-dissociation constant of hydrocyanic acid (HCN)at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents