Exhibit 6A This is a reaction going on in your muscle cells right this very minute: The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics: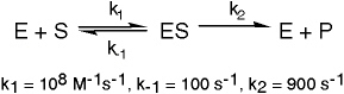 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A.What is the equilibrium constant for the uncatalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
Correct Answer:
Verified
Q24: According to the steady-state assumption
A) the product
Q29: The Michaelis constant is
A) the rate constant
Q42: Exhibit 6A This is a reaction going
Q42: The steady state of an enzyme reaction
Q44: Exhibit 6A This is a reaction going
Q50: Exhibit 6A This is a reaction going
Q53: A Lineweaver-Burk plot is useful in the
Q57: The Michaelis constant determines the Vmax of
Q59: Which of the following statements regarding the
Q62: To study the nature of an enzyme,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents