Which of the following acids would serve as a good buffer for a reaction at pH = 8.0? 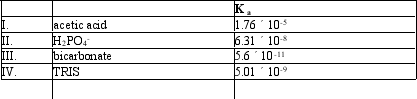
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q50: If a solution has a pH =
Q51: Calculate the final pH of a solution
Q55: The pH of a solution of 0.04
Q61: If the pH of 1 liter of
Q66: Which substance would be the best buffer
Q68: Exhibit 2B Contains information on the pK's
Q73: A buffer solution
A) is used to control
Q76: The ratio of a weak acid and
Q78: The main intracellular buffer system is
A) H3PO4\H2PO4
Q84: Buffers which lack biological activity and are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents