Electromagnetic radiation with a wavelength of 575 nm appears as yellow light to the human eye.The energy of one photon of this light is 3.46 × 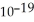 J.Thus,a laser that emits
J.Thus,a laser that emits 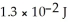 of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
of energy in a pulse of light at this wavelength produces ________ photons in each pulse.
A) 2.7 × 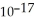
B) 7.8 × 10-24
C) 2.2 × 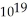
D) 3.8 × 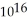
E) 6.5 × 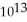
Correct Answer:
Verified
Q94: Place the following types of electromagnetic radiation
Q95: Calculate the frequency of the red light
Q96: Calculate the frequency of the light emitted
Q97: How many photons are contained in a
Q98: Identify the color that has a wavelength
Q100: Identify the color that has a wavelength
Q101: In which orbital below would an electron
Q102: What are the possible values of n
Q103: Which of the following transitions (in a
Q104: How much energy (in kJ)is required to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents