Use the data given below to construct a Born-Haber cycle to determine the bond energy of O2. DH°(kJ)
Na(s) → Na(g) 107
Na(g) → Na⁺(g) + e⁻ 496
O(g) + e⁻ → O⁻(g) -141
O⁻(g) + e⁻ → O2⁻(g) 878
2 Na(s) + 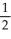 O2(g) → Na2O(s) -416
O2(g) → Na2O(s) -416
2 Na⁺(g) + O2⁻(g) → Na2O(s) -2608
A) 426 kJ
B) 249 kJ
C) 852 kJ
D) 498 kJ
E) 356 kJ
Correct Answer:
Verified
Q1: Place the following in order of decreasing
Q62: Use the data given below to construct
Q63: What is the empirical formula for a
Q64: What is the chemical name for P2I4?
A)
Q65: The energy associated with formation of a
Q66: What is the formula for zinc chloride?
A)
Q68: Use the data given below to construct
Q69: Which of the following reactions is associated
Q70: Use the data given below to construct
Q72: When drawing out Lewis structures,how many electrons
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents