What is the empirical formula of a compound that is 48.6% C,8.2% H,and 43.2% O by mass?
A) 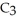
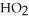
B) 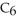
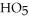
C) 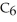
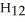
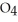
D) 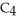
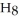
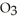
E) 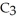
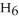
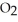
Correct Answer:
Verified
Q49: How many moles of PCl3 contain 3.68
Q87: Which of the following has the smallest
Q130: Determine the mass percent (to the hundredths
Q168: Give the mass percent of hydrogen in
Q169: Which of the following has the greatest
Q170: How many oxygen atoms are there in
Q171: How many cations are there in 30.0
Q175: How many Fe(II)ions are there in 10.0
Q176: What is the mass of 6.78 ×
Q177: How many moles of P I3 contain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents