Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 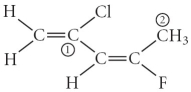
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2 = bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw
Correct Answer:
Verified
Q7: Place the following in order of decreasing
Q16: Place the following in order of increasing
Q20: How many of the following molecules are
Q35: Determine the electron geometry (eg),molecular geometry(mg)and polarity
Q56: Place the following in order of decreasing
Q61: Determine the electron geometry (eg)and molecular geometry
Q68: Determine the electron geometry,molecular geometry and polarity
Q69: Determine the electron geometry (eg),molecular geometry (mg),and
Q70: Determine the electron geometry (eg)and molecular geometry
Q72: Consider the molecule below.Determine the molecular geometry
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents