Consider the molecule below.Determine the hybridization at each of the 3 labeled atoms. 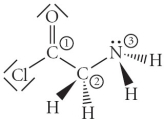
A) 1 = sp2, 2 = sp3, 3 = sp2
B) 1 = sp2, 2 = sp3, 3 = sp3
C) 1 = sp3, 2 = sp3, 3 = sp3
D) 1 = sp3, 2 = sp3, 3 = sp2
E) 1 = sp , 2 = sp , 3 = sp2
Correct Answer:
Verified
Q36: Give the electron geometry (eg),molecular geometry (mg),and
Q37: Describe a pi bond.
A) side by side
Q38: Draw the molecular orbital diagram shown to
Q39: Draw the molecular orbital diagram shown to
Q40: The orbital hybridization on the carbon atoms
Q42: Give the electron geometry,molecular geometry,and hybridization for
Q43: How many of the following molecules have
Q44: How many of the following molecules contain
Q45: Consider the molecule below.Determine the hybridization at
Q46: How many of the following molecules have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents