Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 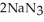 (s) → 2Na(s) +
(s) → 2Na(s) + 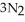 (g) How many grams of sodium azide are required to produce 25.0 g of nitrogen?
(g) How many grams of sodium azide are required to produce 25.0 g of nitrogen?
A) 1.34
B) 0.595
C) 58.0
D) 38.7
E) 87.0
Correct Answer:
Verified
Q49: How many grams of oxygen are formed
Q50: Lithium and nitrogen react to produce lithium
Q51: According to the following balanced reaction,how many
Q52: Lithium and nitrogen react to produce lithium
Q53: If the density of ethanol,C2H5OH,is 0.789 g/mL.How
Q56: Lithium and nitrogen react in a combination
Q58: Balance the chemical equation given below,and determine
Q59: How many moles of CuO can be
Q79: Consider the following reaction.How many moles of
Q93: Strontium phosphate reacts with sulfuric acid to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents