A stock solution of 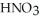 is prepared and found to contain 12.1 M of
is prepared and found to contain 12.1 M of 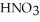 .If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
.If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
A) 0.242
B) 1.65
C) 0.605
D) 605
E) 242
Correct Answer:
Verified
Q21: How many chloride ions are present in
Q70: What is the concentration (M)of C
Q71: What is the concentration (M)of a NaCl
Q73: How many milliliters of a stock solution
Q74: A FeCl3 solution is 0.175 M.How many
Q76: How many grams of Q77: What is the concentration (M)of sodium ions Q78: Pure acetic acid ( Q79: What is the concentration of an AlCl3 Q105: A student prepared a stock solution by![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents