Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 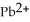 (aq) +
(aq) + 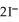 (aq) →
(aq) → 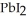 (s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many milliliters of 3.550 M HI(aq) must be added to a solution containing 0.500 mol of
(s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many milliliters of 3.550 M HI(aq) must be added to a solution containing 0.500 mol of 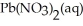 to completely precipitate the lead?
to completely precipitate the lead?
A) 3.55 × 10-3 mL
B) 282 mL
C) 141 mL
D) 0.141 mL
E) 0.282 mL
Correct Answer:
Verified
Q135: Which of the following compounds is an
Q136: The mixing of which pair of reactants
Q138: The mixing of which pair of reactants
Q139: What reagent could be used to separate
Q141: Determine the oxidation state of nitrogen in
Q142: A solution is prepared by adding 1.50
Q144: A solution is prepared by mixing 20.0
Q192: Identify the polyprotic acid.
A) H2SO4
B) HCl
C) LiI
D)
Q205: Determine the oxidation state of Ti in
Q215: When 220.mL of 1.50 × 10-4 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents