Which of the following processes are endothermic?
A) K⁺(g) + I⁻(g) → KI(s)
B) 2 Br(g) → Br2(g)
C) Ca(s) → Ca(g)
D) 2 Na(s) + 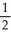 O2(g) → Na2O(s)
O2(g) → Na2O(s)
E) None of the above are endothermic.
Correct Answer:
Verified
Q40: What volume of benzene (C6H6,d= 0.88 g/mL,molar
Q41: Which of the following processes are endothermic?
A)
Q46: Two solutions,initially at 24.69°C,are mixed in a
Q47: Use the standard reaction enthalpies given below
Q49: Which of the following processes are exothermic?
A)
Q59: Use the standard reaction enthalpies given below
Q66: Use the bond energies provided to estimate
Q66: Which of the following processes are exothermic?
A)Cl2(g)→
Q73: Use the bond energies provided to estimate
Q77: Use the bond energies provided to estimate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents