A 5.00-g sample of liquid water at 25.0 C is heated by the addition of 84.0 J of energy.The final temperature of the water is ________°C.The specific heat capacity of liquid water is 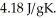
A) 95.2
B) 25.2
C) -21.0
D) 29.0
E) 4.02
Correct Answer:
Verified
Q79: The specific heat capacity of methane gas
Q87: A piece of iron (C=0.449 J/g°C)and a
Q88: Calculate the amount of heat (in kJ)necessary
Q89: A balloon is inflated from 0.0100 L
Q92: Which of the following substances (with specific
Q93: A 6.50-g sample of copper metal at
Q94: The specific heat capacity of solid copper
Q95: A sample of copper absorbs 43.6 kJ
Q96: Give the units of heat capacity.
A) J/°C
B)
Q128: A 50.0-g sample of liquid water at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents