How much heat is absorbed/released when 25.00 g of NH3(g) reacts in the presence of excess 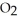 (g) to produce NO(g) and H2O(l) according to the following chemical equation?
(g) to produce NO(g) and H2O(l) according to the following chemical equation?
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l) ΔH° = 1168 kJ
A) 428.6 kJ of heat are absorbed.
B) 428.6 kJ of heat are released.
C) 1715 kJ of heat are absorbed.
D) 1715 kJ of heat are released.
Correct Answer:
Verified
Q18: Calculate the change in internal energy (ΔE)for
Q86: According to the following thermochemical equation,what mass
Q97: When 0.455 g of anthracene,C14H10,is combusted in
Q112: How much energy is evolved during the
Q115: Using the following thermochemical equation,determine the amount
Q117: Which of the following processes is endothermic?
A)
Q118: According to the following reaction,how much energy
Q121: Which of the following processes is exothermic?
A)
Q125: At constant pressure,the combustion of 15.0 g
Q137: When 10.00 moles of H2(g)reacts with 5.000
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents