Hydrogen peroxide decomposes to water and oxygen at constant pressure by the following reaction: 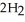
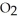 (l) →
(l) → 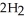 O(l) +
O(l) + 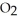 (g) ΔH = -196 kJ Calculate the value of q (kJ) in this exothermic reaction when 4.00 g of hydrogen peroxide decomposes at constant pressure?
(g) ΔH = -196 kJ Calculate the value of q (kJ) in this exothermic reaction when 4.00 g of hydrogen peroxide decomposes at constant pressure?
A) -23.1 kJ
B) -11.5 kJ
C) -0.0217 kJ
D) 1.44 kJ
E) -2.31 × 104 kJ
Correct Answer:
Verified
Q130: Define chemical energy.
Q131: Where does the energy absorbed during an
Q132: Choose the reaction that illustrates ΔH°f for
Q133: If a block of gold requires 1.42
Q134: At constant pressure,the combustion of 6.00 g
Q136: When 50.0 mL of 0.400 M Ca(NO3)2
Q138: Give the temperature and pressure for the
Q138: Identify a substance that is NOT in
Q139: It takes 18.2 kJ of energy to
Q140: Sodium metal reacts with water to produce
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents