Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + 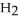
 (aq) → Zn
(aq) → Zn 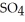 (aq) +
(aq) + 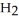 (g)
(g)
In an experiment,225 mL of wet 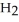 is collected over water at 27°C and a barometric pressure of
is collected over water at 27°C and a barometric pressure of 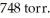 How many grams of Zn have been consumed?
How many grams of Zn have been consumed?
The vapor pressure of water at 27°C is 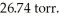
A) 4.79 × 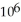
B) 0.567
C) 567
D) 431
E) 4.31 × 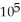
Correct Answer:
Verified
Q61: In a container containing CO, H2, and
Q121: A mixture of 0.220 moles CO,0.350 moles
Q122: Give the gas that is the lowest
Q124: A gas mixture contains CO,Ar and H2.What
Q125: A mixture of N2,O2 and He have
Q128: The concentration of carbon monoxide in a
Q130: The concentration of ozone in a sample
Q131: A sample of air from a home
Q154: A balloon contains 0.76 mol N2,0.18 mol
Q155: What is the total pressure in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents