Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + 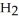
 (aq) → Zn
(aq) → Zn 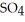 (aq) +
(aq) + 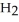 (g)
(g)
In an experiment,201 mL of wet 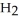 is collected over water at 27°C and a barometric pressure of
is collected over water at 27°C and a barometric pressure of 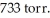 The vapor pressure of water at 27°C is 26.74 torr.The partial pressure of hydrogen in this experiment is ________ atm.
The vapor pressure of water at 27°C is 26.74 torr.The partial pressure of hydrogen in this experiment is ________ atm.
A) 0.929
B) 706
C) 0.964
D) 760
E) 1.00
Correct Answer:
Verified
Q34: A mixture of 0.220 moles CO,0.350 moles
Q99: The mole fraction of oxygen in dry
Q117: The mole fraction of argon in dry
Q130: The concentration of ozone in a sample
Q131: A sample of air from a home
Q134: The concentration of water vapor in a
Q136: A mixture of Ar,Ne and He has
Q138: The mole fraction of carbon dioxide in
Q145: In the laboratory,hydrogen gas is usually made
Q149: A 0.600 g sample containing Ag2O and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents