Calcium hydride ( 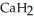 ) reacts with water to form hydrogen gas:
) reacts with water to form hydrogen gas: 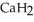 (s) +
(s) + 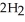 O(l) →
O(l) → 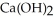 (aq) +
(aq) + 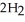 (g) How many grams of
(g) How many grams of 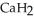 are needed to generate 48.0 L of
are needed to generate 48.0 L of 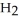 gas at a pressure of 0.888 atm and a temperature of 32°C?
gas at a pressure of 0.888 atm and a temperature of 32°C?
A) 50.7
B) 0.851
C) 143
D) 35.8
E) 71.7
Correct Answer:
Verified
Q127: How many grams of calcium hydride are
Q147: Rank the following in order of decreasing
Q147: What is the temperature of NO2 gas
Q148: Identify the gas particle that travels the
Q152: The action of some commercial drain cleaners
Q153: When 14.0 g of zinc metal reacts
Q154: How many grams of zinc metal are
Q158: If NO and NH3 are allowed
Q159: Which of the following samples will have
Q160: How many grams of XeF6 are required
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents