Consider the phase diagram shown.Choose the statement below that is TRUE. 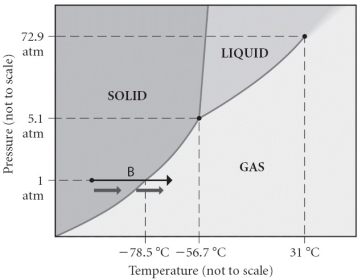
A) The triple point of this substance occurs at a temperature of 31°C.
B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist.
C) The solid phase of this substance is higher in density than the liquid phase.
D) The line separating the solid and liquid phases represents the ΔHvap.
E) None of the above are true.
Correct Answer:
Verified
Q65: Which of the following compounds has dipole-dipole
Q66: Consider the phase diagram shown.What is the
Q67: The heat of vaporization of acetone is
Q69: An unknown substance has a boiling point
Q73: Consider the phase diagram shown.What is the
Q74: List the compounds in decreasing boiling point
Q111: The normal boiling point for H2Se
Q115: What is the strongest type of intermolecular
Q118: In liquid propanol,CH3CH2CH2OH Which intermolecular
Q142: Which is expected to have the largest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents