Based on the figure above,the boiling point of diethyl ether under an external pressure of 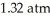 is ________ °C.
is ________ °C.
A) 10
B) 20
C) 30
D) 40
E) 0
Correct Answer:
Verified
Q78: Define triple point.
A) The temperature, pressure, and
Q79: What is the strongest type of intermolecular
Q80: Calculate the energy that is required to
Q81: Based on the figure above,the boiling point
Q82: Identify the substance with the highest viscosity.
A)
Q84: What type of intermolecular force causes the
Q85: Choose the compound that exhibits hydrogen bonding
Q86: Choose the substance with the highest surface
Q88: Choose the substance with the lowest surface
Q103: Identify the compound that does not have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents