Consider the phase diagram shown below.If you start at 0.75 atm and 0 °C,and move to 0.10 atm and 25C,you will move from the ________ phase to the ________ phase. 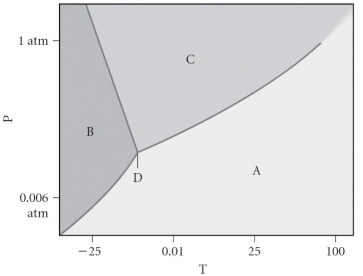
A) liquid, gas
B) gas, gas
C) liquid, liquid
D) gas, solid
E) liquid, solid
Correct Answer:
Verified
Q101: Why do O,F and N,when bonded to
Q107: Define viscosity.
Q123: Define boiling point of a liquid.
Q124: Define volatile.
Q125: Why does the temperature of a substance
Q126: Sketch the phase diagram of benzene.Make sure
Q127: Define dynamic equilibrium.
Q128: Why is the ΔHvap higher than ΔHfus
Q128: What is the heat of vaporization of
Q130: Choose the compound that exhibits hydrogen bonding
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents