Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 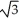 .
.
A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3
Correct Answer:
Verified
Q5: Identify the type of solid for diamond.
A)
Q10: Identify the type of solid for gold.
A)
Q13: When an X-ray beam strikes the surface
Q15: Determine the radius of an Al atom
Q16: A common use of polyurethane is
A) spray-on
Q17: When an X-ray beam with a wavelength
Q20: Vanadium crystallizes in a body centered cubic
Q22: Give the coordination number for a body-centered
Q149: Lithium crystallizes in a body-centered cubic structure.What
Q150: Nickel has a face-centered cubic structure and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents