Write a balanced reaction for which the following rate relationships are TRUE. Rate = - 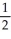
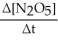 =
= 
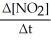 =
= 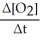
A) 2 N2O5 → 4 NO2 + O2
B) 4 NO2 + O2 → 2 N2O5
C) 2 N2O5 → NO2 + 4 O2
D)  NO2 + O2 →
NO2 + O2 → 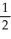 N2O5
N2O5
E) 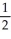 N2O5 →
N2O5 →  NO2 + O2
NO2 + O2
Correct Answer:
Verified
Q3: The reaction,2A + 2B → C +
Q4: Determine the rate law and the value
Q5: Give the characteristic of a zero-order reaction
Q5: What are the units of k in
Q6: Given the following balanced equation,determine the rate
Q7: What are the units of k in
Q8: What are the units of k in
Q9: Write a balanced reaction for which the
Q10: What are the units of k in
Q11: Identify the methods used to monitor a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents