Write a balanced reaction for which the following rate relationships are TRUE. Rate = 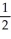
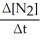 =
= 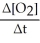 = -
= - 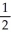
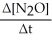
A) 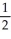 N2 + O2 →
N2 + O2 → 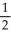 N2O
N2O
B) 2 N2O → 2 N2 + O2
C) N2O → N2 + 2 O2
D) 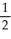 N2O →
N2O → 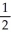 N2 + O2
N2 + O2
E) 2 N2 + O2 → 2 N2O
Correct Answer:
Verified
Q3: Give the characteristic of a second-order reaction
Q4: Determine the rate law and the value
Q5: What are the units of k in
Q6: Given the following balanced equation,determine the rate
Q7: What are the units of k in
Q8: What are the units of k in
Q10: What are the units of k in
Q11: Identify the methods used to monitor a
Q12: Given the following balanced equation,determine the rate
Q14: What is the overall order of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents