Derive an expression for a "1/3-life" for a first-order reaction.
A) 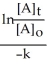
B) 
C) 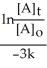
D) 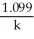
E) 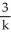
Correct Answer:
Verified
Q22: The first-order decomposition of N2O5 at 328
Q25: The second-order decomposition of NO2 has a
Q45: Biological catalysts that increase the rates of
Q46: If the activation energy for a given
Q48: A reaction is followed and found to
Q49: In the hydrogenation of double bonds,a catalyst
Q55: A reaction occurs via the following sequence
Q62: Given the following balanced equation,determine the rate
Q63: A reaction is found to have an
Q63: Given the following balanced equation,determine the rate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents