The rate of disappearance of HBr in the gas phase reaction 2HBr(g) → H2(g) + Br2(g)
Is 0.301 M 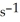 at 150°C.The rate of appearance of
at 150°C.The rate of appearance of 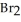 is ________ M
is ________ M 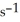 .
.
A) 1.66
B) 0.151
C) 0.0906
D) 0.602
E) 0.549
Correct Answer:
Verified
Q42: Identify an heterogeneous catalyst.
A) CFCs with ozone
B)
Q45: Biological catalysts that increase the rates of
Q62: Given the following balanced equation,determine the rate
Q63: Given the following balanced equation,determine the rate
Q65: A graph of 1/[A] vs.t gives a
Q69: The first-order decomposition of ammonium nitrate has
Q70: The rate of disappearance of HBr in
Q71: Given the following balanced equation,determine the rate
Q72: Reaction rate can change with
A) temperature.
B) the
Q77: Identify an homogeneous catalyst.
A)SO2 over vanadium (V)oxide
B)Pd
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents