Express the equilibrium constant for the following reaction. 16 CH3Cl(g) + 8 Cl2(g) ⇔ 16 CH2Cl2(g) + 8 H2(g)
A) K = 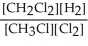
B) K = 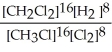
C) K = 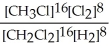
D) K = 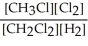
E) K = 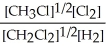
Correct Answer:
Verified
Q2: The equilibrium constant is given for one
Q3: What is Δn for the following equation
Q5: Chemical equilibrium is the result of
A) all
Q6: Equilibrium in which rate of the forward
Q6: Which of the following statements is FALSE?
A)
Q9: What is Δn for the following equation
Q12: Give the direction of the reaction,if K
Q12: Give the direction of the reaction,if K
Q19: The equilibrium constant is given for two
Q20: What is △n for the following equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents