What is the pH of a 0.50 M H2Se solution that has the stepwise dissociation constants Ka1 = 1.3 × 10-4 and 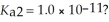
A) 2.09
B) 3.89
C) 4.19
D) 5.57
Correct Answer:
Verified
Q143: Describe the relationship between molecular structure and
Q144: What does the term amphoteric mean?
Q146: Do both protons ionize instantaneously from a
Q147: What is the pOH of a 0.0020
Q151: What is the selenide ion concentration [Se2-]
Q151: Describe a molecule that can be a
Q153: Calculate the concentration of bicarbonate ion,HCO3-,in a
Q154: Determine the Ka of an acid that
Q155: What is the difference between a strong
Q157: Calculate the pH of a 0.80 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents