How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 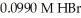 to the equivalence point?
to the equivalence point?
A) 29.5 mL
B) 0.332 mL
C) 4.57 mL
D) 0.208 mL
E) 21.2 mL
Correct Answer:
Verified
Q24: Formic acid (HCO2H,Ka = 1.8 × 10-4)is
Q124: What is the pH of a solution
Q125: A 25.0 mL sample of 0.150 M
Q126: What is the pH of a solution
Q127: What volume of 5.00 × 10-3 M
Q129: Identify the indicator that has two endpoints.
A)
Q131: What is the approximate pH at the
Q132: A 25.0-mL sample of 0.150 M benzoic
Q133: What is the pH of the resulting
Q150: Sodium hypochlorite,NaOCl,is the active ingredient in household
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents