Phosphorous and chlorine gases combine to produce phosphorous trichloride: P2(g) + 3Cl2(g) → 2PCl3(g)
ΔG° at 298 K for this reaction is -642.9 kJ/mol.The value of ΔG at 298 K for a reaction mixture that consists of 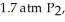
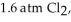 and
and 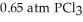 is
is
A) -39.0
B) -4.19 × 103
C) -7.59 × 103
D) -711.4
E) -649.8
Correct Answer:
Verified
Q82: Determine ΔG°rxn for the following reaction at
Q98: Consider the following reaction at constant P.Use
Q99: Identify the compound with the standard free
Q100: Given the following equation, C3H8(g)+ 5 O2(g)→
Q104: For a particular reaction,the value of ∆H
Q106: Why is heating your home with gas
Q108: In the Haber process,ammonia is synthesized from
Q110: The value of ΔG° at 141.0°C for
Q112: Why can't we say that a spontaneous
Q123: How is a nonspontaneous process made spontaneous?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents