The most useful ore of aluminum is bauxite,in which Al is present as hydrated oxides, 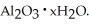 The number of kilowatt-hours of electricity required to produce
The number of kilowatt-hours of electricity required to produce 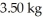 of aluminum from electrolysis of compounds from bauxite is ________ when the applied emf is
of aluminum from electrolysis of compounds from bauxite is ________ when the applied emf is 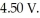
A) 0.0213
B) 0.0469
C) 31.3
D) 15.6
E) 46.9
Correct Answer:
Verified
Q37: For a galvanic cell that uses the
Q71: A voltaic cell is constructed with two
Q123: The gas OF2 can be produced from
Q124: A cell consists of a Sn4+|| Sn2+
Q127: How many seconds are required to produce
Q128: The town of Natrium,West Virginia,derives its name
Q129: CuHow many electrons are transferred in the
Q130: The standard emf for the cell using
Q145: What is the difference between a voltaic
Q181: How long must a constant current of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents