Complete the following equation of transmutation. 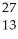 Al +
Al + 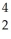 He →
He → 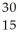 P + ______
P + ______
A) 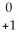 e
e
B) 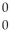 γ
γ
C) 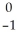 e
e
D) 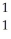 H
H
E) 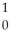 n
n
Correct Answer:
Verified
Q24: Neptunium-239 has a half-life of 2.35 days.How
Q89: Potassium-40 decays to argon-40 with a half-life
Q102: Strontium-90 is a byproduct in nuclear reactors
Q107: Give the maximum age that can be
Q109: Complete the following equation of transmutation.
Q110: Above what atomic number are there no
Q114: The radioactive decay of _ is the
Q114: If we start with 1.000 g of
Q115: A rock contains 0.112 mg of lead-206
Q116: Carbon-11 is used in medical imaging.The half-life
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents