Two students are tasked with determining the milligrams of chloride in a simulated soil sample.Both students extract chloride into aqueous solution and perform triplicate titration with silver nitrate solution and dichlorofluorescein indicator.The students report the following results to their laboratory instructor. 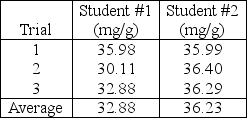 If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #1's work exhibits____________________ student #2.
If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #1's work exhibits____________________ student #2.
A) higher accuracy and higher precision than
B) higher accuracy and lower precision than
C) lower accuracy and lower precision than
D) lower accuracy and higher precision than
E) results impossible to differentiate from
Correct Answer:
Verified
Q1: A mass of approximately 10 grams is
Q3: The density of a solution is determined
Q4: _ is a consistent error that can
Q5: For significant digits and calculations,the following statements
Q6: For the paired statements below,which are TRUE
Q7: Calculate the molar mass of diethyl ether,CH3CH2OCH2CH3.(C
Q8: Calculate the mass of a concrete slab
Q9: _ cannot be eliminated but it may
Q10: Calculate the mass of a heterogeneous mixture
Q11: The distance between two cities is measured
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents