The concentration and absorbance data below was collected for compound X.The least squares line for concentration versus absorbance is y = 6.83x + 0.0881.If a sample of compound X of unknown concentration has a corrected average absorbance of 0.6521 (three replicate measurements)calculate the concentration compound X and the uncertainty in the concentration.
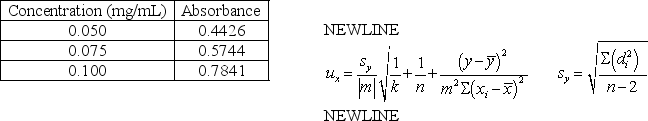 Concentration (mg/mL)
Concentration (mg/mL)
Absorbance
0.050
0.4426
0.075
0.5744
0.100
0.7841
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q12: A technician determines the concentration of calcium
Q13: Calculate the mean and standard deviation
Q14: The absorbance for a dye sample
Q15: Doubling the number of calibration curve data
Q16: The average percent purity for a batch
Q17: Which of the following are true for
Q18: The molarity of a sodium hydroxide solution
Q19: A researcher has developed a new analytical
Q20: For the statements below,which is(are)TRUE for confidence
Q22: The molarity of a sodium hydroxide solution
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents