The pH of a solution composed of NaO2CCO2H and NaCl must be determined.To get a more complete picture of the equilibrium,the mass balance equation for total oxalate is written.Which is the ONLY valid mass balance equation?
A) 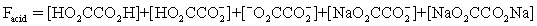
B) 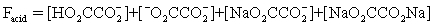
C) 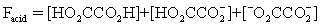
D) 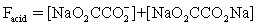
E) 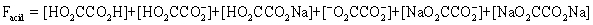
Correct Answer:
Verified
Q1: A student is tasked to determine the
Q2: Calculate the concentration of each fumaric acid,HO2CCHCHCO2H,species
Q4: A solution is 0.200 M in acetic
Q5: Which of the fractional composition equations is
Q6: The mean fraction of protons, 
Q7: The pH of a solution composed of
Q8: The mass balance equation for the solubility
Q9: Calculate the pH for a buffer that
Q10: Which is the correct effective solubility constant
Q11: Express [Ca2+] in terms [H+] for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents