For the Reaction Below,the Oxidizing Agent Is____________________ and It____________________ Electrons,and
For the reaction below,the oxidizing agent is____________________ and it____________________ electrons,and the reducing agent is____________________ and it____________________ electrons. 8 H+ + 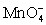 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
A) 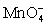
;gains 5;Cu+;loses 1
B) H+;loses 1;Cu+;gains 1
C) H+;gains 1;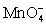
;loses 5
D) Cu+;loses 1;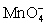
;gains 1
E) Cu+;loses 1;H+;gains 1
Correct Answer:
Verified
Q5: Calculate Eo and K for the cell
Q6: For the cell diagram below write out
Q7: All of the following are true for
Q8: Calculate the standard potential when the two
Q9: A bromide probe is constructed from a
Q11: A galvanic cell is at equilibrium
Q12: Which is true when electrochemical cells are
Q13: Which is NOT correct for when the
Q14: Eo' is defined as:
A)the formal half-cell
Q15: The half-cells below are connected via a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents