Calculate the standard potential when the two half-cells below are connected via a salt bridge and potentiometer.Which species are the reducing agent and oxidizing agent? 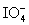 + 2 H+ + 2 e- ⇋
+ 2 H+ + 2 e- ⇋ 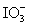 + H2O E° = 1.589 V
+ H2O E° = 1.589 V
Mg(OH)2 (s)+ 2 e- ⇋ Mg (s)+ 2 OH- E° = -2.690 V
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: Calculate E°' for the half-cell below. O2
Q4: For the silver half-reaction,Ag+ + 1 e-
Q5: Calculate Eo and K for the cell
Q6: For the cell diagram below write out
Q7: All of the following are true for
Q9: A bromide probe is constructed from a
Q10: For the reaction below,the oxidizing agent is_
Q11: A galvanic cell is at equilibrium
Q12: Which is true when electrochemical cells are
Q13: Which is NOT correct for when the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents