Calculate E for the titration of 50.00 mL of 0.100 M Ag+ when 50.00 mL of 0.150 M Cl− is added.Silver wire is the indicator electrode and the saturated Ag-AgCl electrode is the reference electrode.E = 0.197 V for the saturated Ag-AgCl electrode, 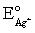 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
Correct Answer:
Verified
Q2: An electrode has a potential of 1.201
Q3: The potential of a silver electrode is
Q4: The selectivity coefficient for a fluoride ion-selective
Q5: To compensate for a complex or unknown
Q6: A sodium selective electrode increased in voltage
Q8: A 0.100 M KCl solution is placed
Q9: For indicator electrodes,which statements are true?
I Indicator
Q10: Which is NOT an error associated with
Q11: The_ gives the relative response of an
Q12: An electrode with a fixed potential is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents