A 50.00 mL aliquot of a water sample is reacted with excess potassium iodide in acidic solution to generate 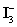 .Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the
.Carbon dioxide is bubbled through the solution to remove nitrogen monoxide generated.The water sample is transferred to a 500 mL volumetric flask and diluted to volume.A 50.00 mL aliquot is then titrated against 1.092 x 10−4 M thiosulfate,requiring 15.48 mL to reach the starch end point.What is the 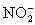 concentration in ppm? Assume solution density of 1.000 g/mL.
concentration in ppm? Assume solution density of 1.000 g/mL. 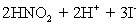 ⇋
⇋ 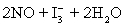
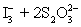 ⇋
⇋ 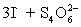
Correct Answer:
Verified
Q10: Calculate the potential for a redox titration
Q11: Which of the statements below are true
Q12: In addition to redox indicators and starch-iodine
Q13: Which statements are true for redox indicators?
I.The
Q14: Which are TRUE for the use of
Q16: Cerium(IV)is a strong oxidizing agent commonly used
Q17: For redox titrations,the oxidation state of the
Q18: A Ce4+ standard solution is prepared with
Q19: A permanganate solution is prepared by dissolving
Q20: Which is NOT true for oxidation with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents